12 January 2016
+SpaceX has published a recap video of their historic Falcon 9 launch including a closeup of the first stage rocket booster vertically landing in Cape Canaveral, FL. The rocket deploys four landing struts as it approaches the ground while being slowed by the main engine. The ability to re-use the most expensive component of a launch vehicle promises to greatly reduce both the time and cost of orbital delivery.
Source: https://youtu.be/ANv5UfZsvZQ (SpaceX)
#ScienceGIF #Science #GIF #SpaceX #Falcon #Falcon9 #Space #OuterSpace #Astronomy #Aeronautical #Engineering #Landing #Rocket #Aerospace
View Original Post on Google+

9 January 2016
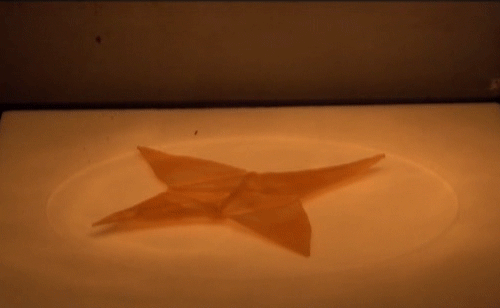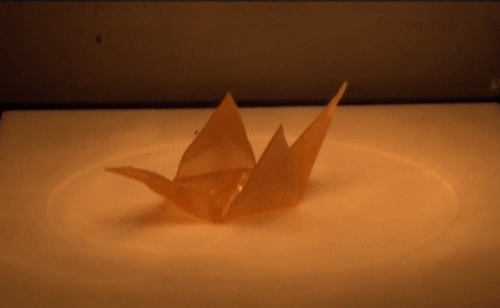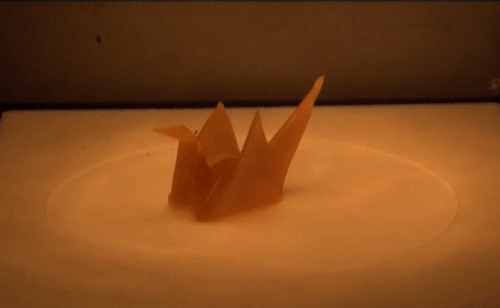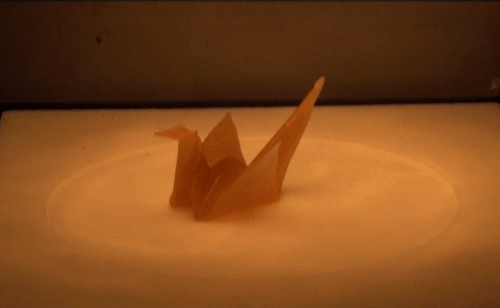
Material scientists have created a thermomechanical polymer that can be “programmed” to perform complex folding. Here, a sheet of the polymer folds itself into an origami bird when exposed to heat. The shape memory polymer can undergo the transition many times without losing functionality.
Source: http://advances.sciencemag.org/content/2/1/e1501297
#ScienceGIF #Science #GIF #Polymer #Physics #Chemistry #Elasticity #Plasticity #Thermal #Network #Memory
View Original Post on Google+
8 January 2016
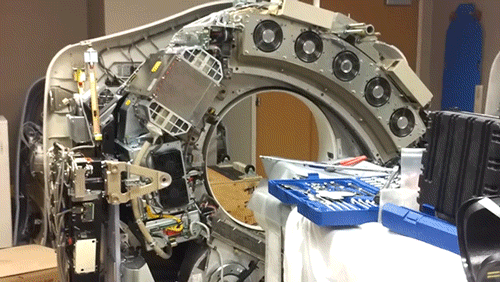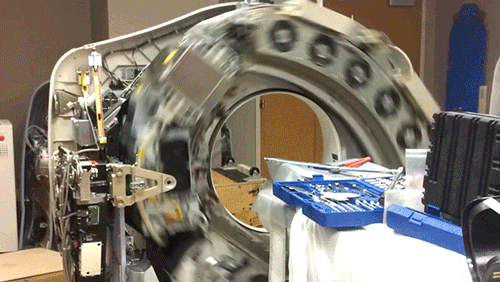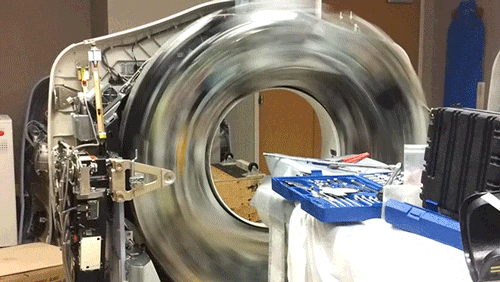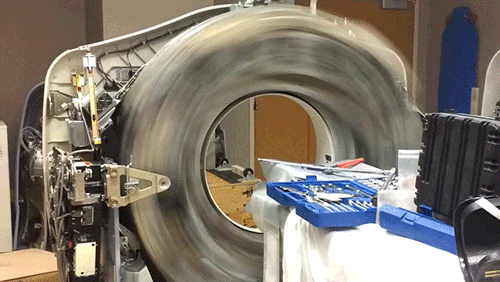
X-Ray Computerized Tomography (CT) produces cross-sectional images of patients by taking X-ray images from different angles and merging them together using a computer. In order to acquire the imagery from different angles, the scanner is rapidly rotated around the patient. Here, the cowling of a CT scanner has been removed to reveal the collection of hardware that is rotated around the central bore hole where the patient is placed.
The CT scanner is comprised of two primary components: the X-ray source and the X-ray detector. The two are located directly across from one another so that X-rays passing through the patient are captured by the detector. Rather than emit a linear beam of X-rays, the source typically emits a fan-shaped beam so that a larger volume of the patient can be imaged. This requires the use of a linear detector array (component with the 5 cooling fans) that captures the expanding beam of X-rays.
Source: https://youtu.be/cjtHNxf01tQ
#ScienceGIF #Science #GIF #CT #CAT #ComputerizedTomography #Medical #MedicalImaging #XRay #Computer #Hardware #Imaging #Technology
View Original Post on Google+
6 January 2016
If you’ve ever dropped water onto a hot surface, you’ve probably noticed that it skitters around rather than just staying in place. It also doesn’t immediately evaporate into water vapor. This occurs because of a physical phenomenon known as the Leidenfrost Effect.
When a liquid comes in contact with a hot surface that is significantly above its boiling point, the bottom portion of the droplet vaporizes immediately. The resulting layer of vapor acts as a cushion for the remainder of the liquid water and prevents direct contact with the hot surface. Because steam is a poor thermal conductor, further heat transfer between the hot surface and liquid water is slowed dramatically. This prevents the remainder of the liquid water from immediately evaporating. Since the droplet is resting on a cushion of vapor, it can easily slide around the surface of the pan.
The temperature necessary for this effect to occur (Leidenfrost Point) with water is 193°C or 379°F.
Source: https://youtu.be/mZenCYF1IpM
#ScienceGIF #Science #GIF #Physics #Water #Hot #Stove #Boiling #Vapor #Droplet #Leidenfrost #Temperature #Matter #State #Liquid #Solid #Gas #Vapor #Steam
View Original Post on Google+

5 January 2016
pH indicators are used to visually detect variations in acidity/basicity via changing color. In this case, two indicators (bromothymol and phenolphthalein) are combined in a solution of isopropanol and ethanol. When a chunk of sodium is introduced, it reacts with the alcohol mixture to form an alkaline environment and therefore produces a color change. However, because isopropanol has a high viscosity, the propagation is very slow, which results in the flower-like distribution of color around the sodium.
Source: https://youtu.be/RQzFPQ5sy-w
#ScienceGIF #Science #GIF #Indicator #Chemical #ChemicalFlower #Aklaline #Ethanol #Isopropanol #Sodium #Colorful #Reaction
View Original Post on Google+

29 December 2015
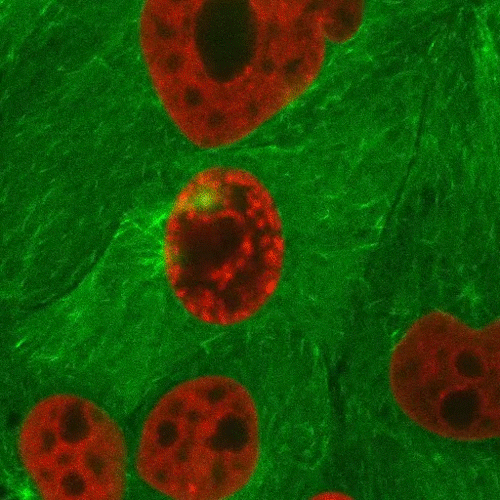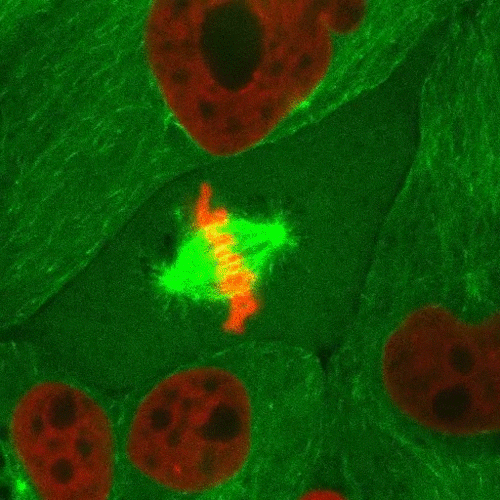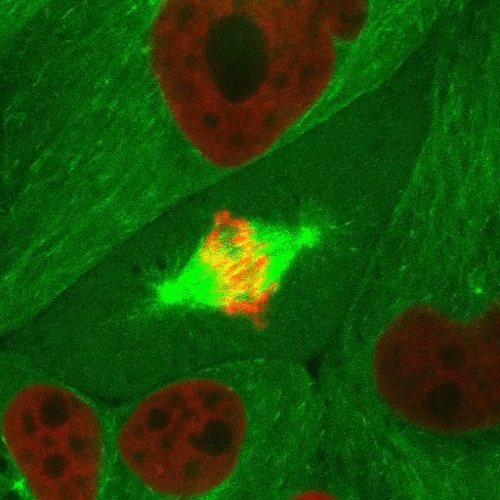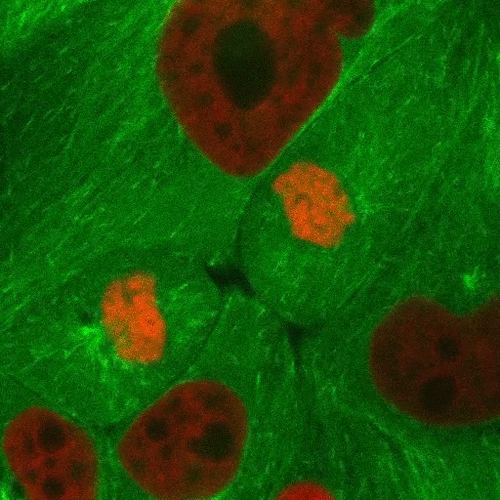
Mitosis is a part of the cell cycle in which chromosomes in a cell nucleus are separated into two identical sets and incorporated into the nuclei of the resulting daughter cells.
As the parent cell prepares to replicate, the chromosomes are duplicated, condensed, and aligned along the middle of the cell (metaphase plate). Spindle fiber microtubules extend from opposite sides of the cell and attach to the chromosomes, which are then pulled apart and form the new nuclei. The cell then divides in half through a process called cytokinesis to form the two daughter cells.
This confocal fluorescence microscopy sequence depicts the process with the chromosomes shown in red and the microtubules shown in light green. Fluorescent proteins are used to selectively label the different components of the cell for study.
Source: http://www.microscopyu.com/moviegallery/c1si/mitosiseb3/index.html (Nikon)
#ScienceGIF #Science #GIF #Biology #Mitosis #Cell #Division #Replication #Chromatids #Spindle #Microtubules #Fluorescence #Microscopy #Nikon #Metaphase #Chromosomes
View Original Post on Google+
28 December 2015
Molecular oxygen has two unpaired electrons and therefore possesses paramagnetic properties. In the presence of an external magnetic field, paramagnetic materials form an induced internal magnetic field aligned with the external field. This occurs because the spin of the unpaired electrons aligns with the external magnetic field. The result is the liquid oxygen becoming attracted to the magnet and sticking between the two poles.
If this same experiment were performed with molecular nitrogen, no such attraction would occur because each of its electrons are paired.
Source: https://youtu.be/Lt4P6ctf06Q
#ScienceGIF #Science #GIF #Magnetism #Oxygen #LiquidOxygen #Magnet #Paramagnetism #Electrons #Orbitals #Chemistry #Physics
View Original Post on Google+
27 December 2015
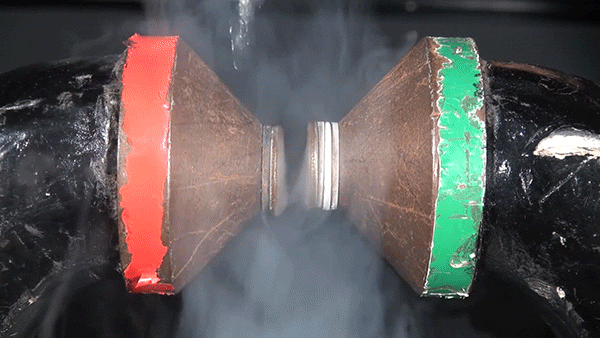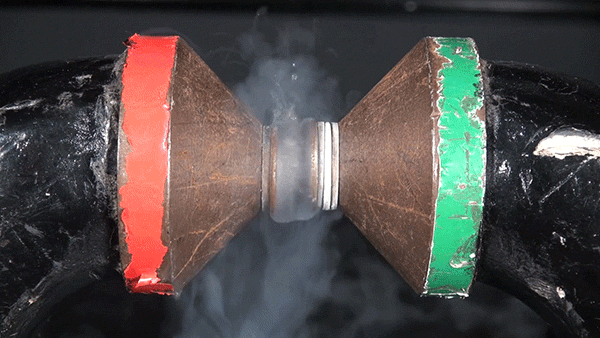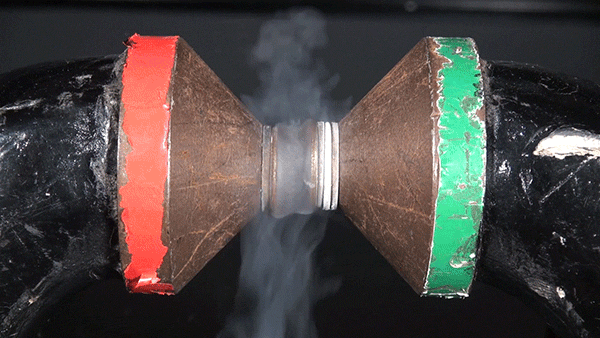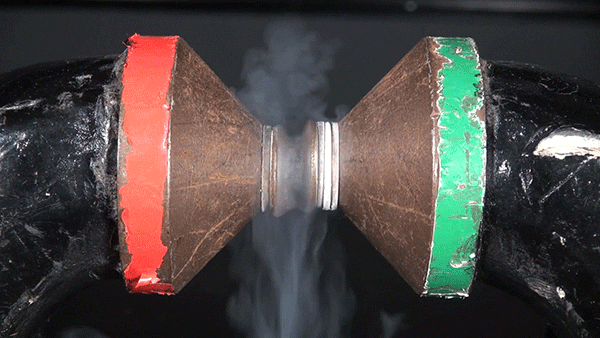
Sodium chloride, commonly known as table salt, is a notoriously inert substance. However, its constituent elements, sodium (Na) and chloride (Cl), are both extremely reactive and dangerous. The reaction between the two is highly exothermic and results in a cloud of sodium chloride smoke.
In this demonstration, the glass container is filled with chlorine gas. Sodium metal is heated over a Bunsen burner and submerged into the gas to initiate the reaction.
Source: https://youtu.be/d2geiGKFveE (UCBerkeley)
#ScienceGIF #Science #GIF #Reaction #SodiumChloride #Salt #Reaction #Chemistry #Exothermic #Chlorine #Sodium
View Original Post on Google+

26 December 2015
Tornadoes are violently rotating columns of air that typically form from the base of a cumuliform cloud and extend downwards to the surface of the earth. They form within mesocyclones (area of organized rotation) commonly found in supercell thunderstorms. The convergence of cool, moist air in the downdraft with warm air in the updraft causes the initial rotation.
Most tornadoes have wind speeds less than 110mph, reach a diameter around 250ft, and only travel a few miles before dissipating. Extreme tornadoes can attain wind speeds upwards of 300mph, stretch more than two miles in diameter, and cause devastation for dozens of miles. Tornadoes have been observed on every continent except for Antarctica with the majority occurring in the “Tornado Alley” region of North America.
Source: https://youtu.be/7KDz6dGQ5RE (National Geographic)
#ScienceGIF #Science #GIF #Weather #Tornado #Atmosphere #Air #Destruction #Damage #NationalGeographic #Meteorology
View Original Post on Google+

25 December 2015
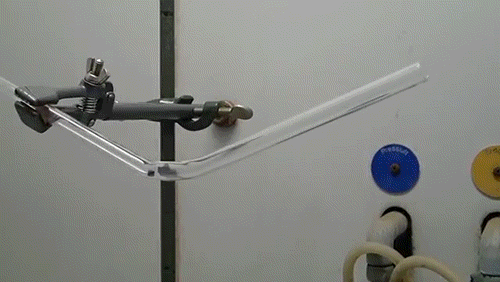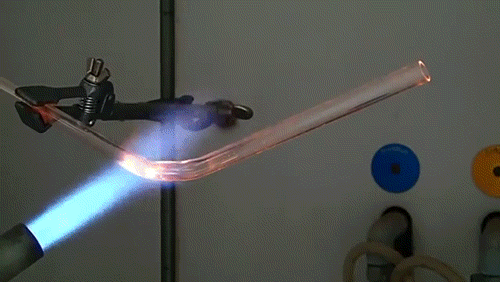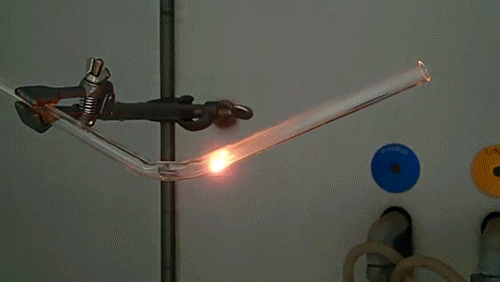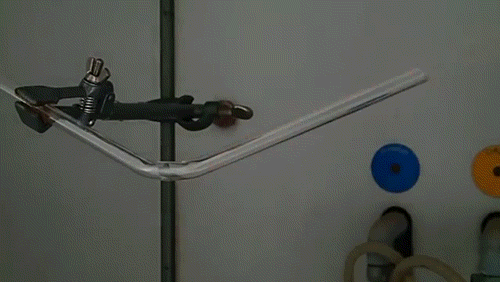
Despite their reputation for being the hardest naturally-occurring material on the planet, diamonds can easily be burned under the correct conditions. While heating with a conventional acetylene torch can ignite a diamond, the lack of oxygen in the atmosphere severely limits the reaction.
Here, a low-quality diamond is placed into a glass tube flowing oxygen gas. The diamond is heated using the torch until it ignites and begins to glow. As the diamond burns, its carbon molecules combine with the oxygen to form carbon monoxide and carbon dioxide. The burning proceeds until the diamond has evaporated away into gases.
The burning of a diamond was used by Antoine Lavoisier in the late 18th century to argue that diamonds were composed of carbon and provided important insight into the process of combustion and the existence of elemental oxygen.
Source: https://youtu.be/PoxyZjwd17k
#ScienceGIF #Science #GIF #Diamond #Carbon #Oxygen #Burning #CarbonDioxide #CarbonMonoxide
View Original Post on Google+