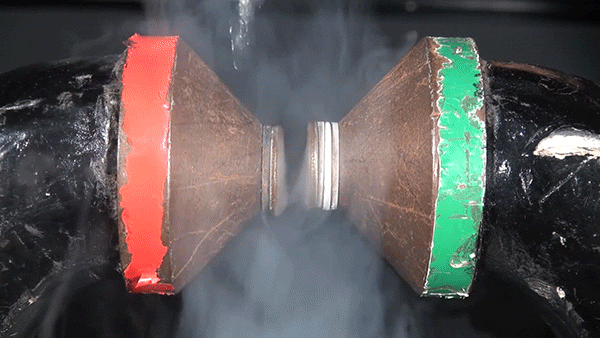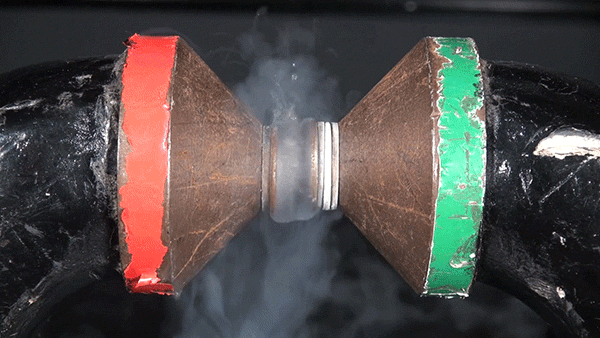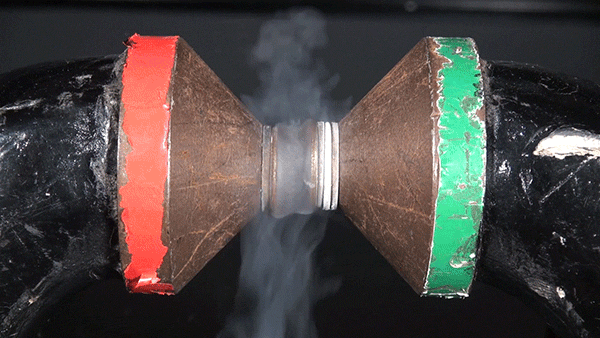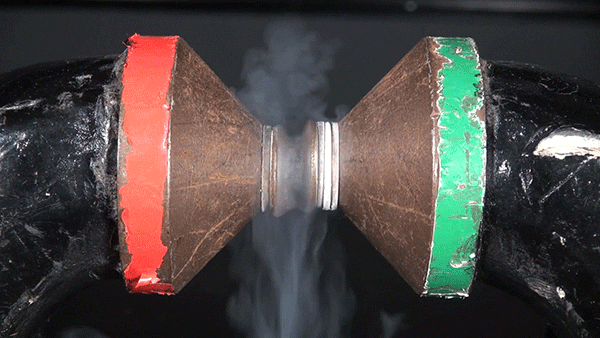
Molecular oxygen has two unpaired electrons and therefore possesses paramagnetic properties. In the presence of an external magnetic field, paramagnetic materials form an induced internal magnetic field aligned with the external field. This occurs because the spin of the unpaired electrons aligns with the external magnetic field. The result is the liquid oxygen becoming attracted to the magnet and sticking between the two poles.
If this same experiment were performed with molecular nitrogen, no such attraction would occur because each of its electrons are paired.
Source: https://youtu.be/Lt4P6ctf06Q
#ScienceGIF #Science #GIF #Magnetism #Oxygen #LiquidOxygen #Magnet #Paramagnetism #Electrons #Orbitals #Chemistry #Physics View Original Post on Google+