Despite their reputation for being the hardest naturally-occurring material on the planet, diamonds can easily be burned under the correct conditions. While heating with a conventional acetylene torch can ignite a diamond, the lack of oxygen in the atmosphere severely limits the reaction.
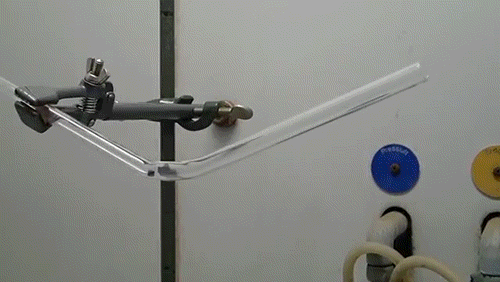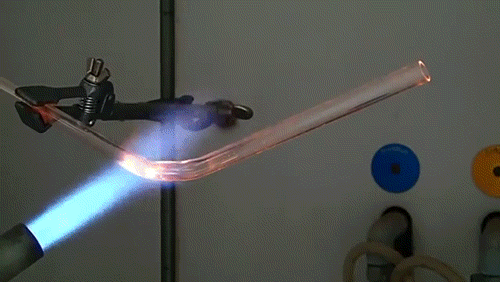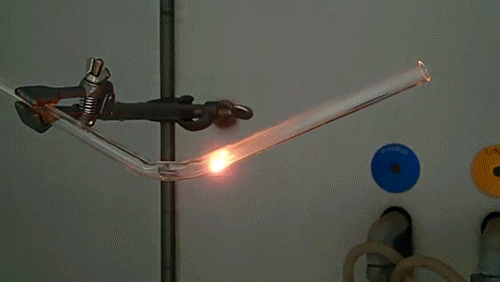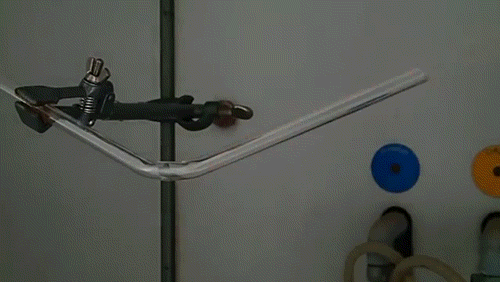
Here, a low-quality diamond is placed into a glass tube flowing oxygen gas. The diamond is heated using the torch until it ignites and begins to glow. As the diamond burns, its carbon molecules combine with the oxygen to form carbon monoxide and carbon dioxide. The burning proceeds until the diamond has evaporated away into gases.
The burning of a diamond was used by Antoine Lavoisier in the late 18th century to argue that diamonds were composed of carbon and provided important insight into the process of combustion and the existence of elemental oxygen.
Source: https://youtu.be/PoxyZjwd17k
#ScienceGIF #Science #GIF #Diamond #Carbon #Oxygen #Burning #CarbonDioxide #CarbonMonoxide View Original Post on Google+