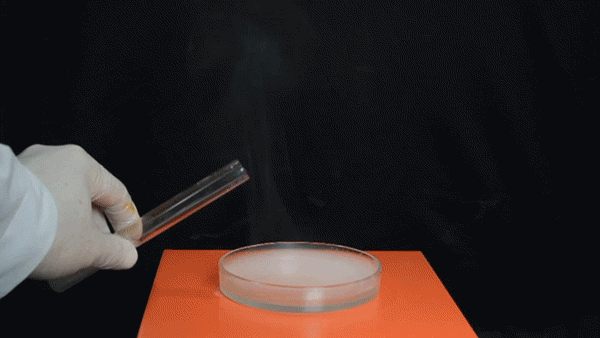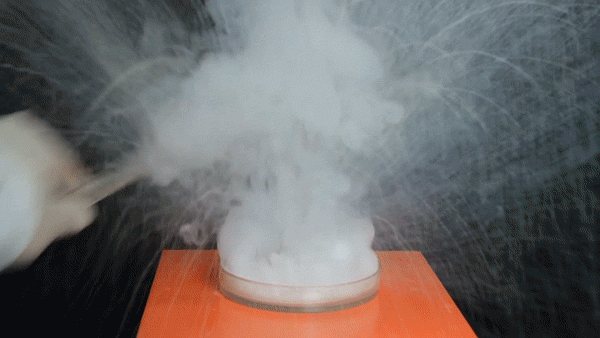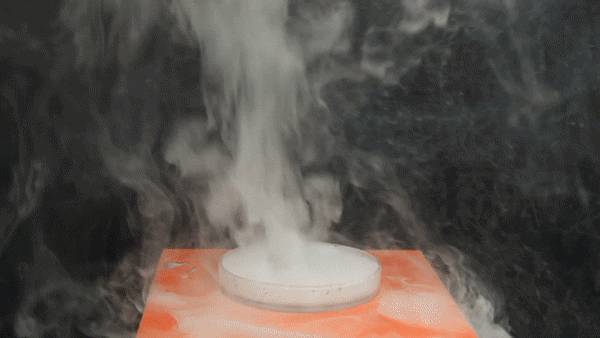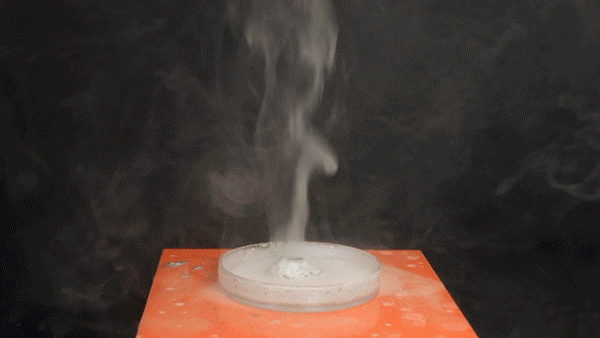
Sodium Borohydride (NaBH4) is a broadly used reducing agent most commonly found as a white powder. Tin Tetrachloride (SnCl4) is a colorless hygroscopic liquid, which fumes on contact with air, and is predominantly used in organotin chemistry. It was also used as a chemical weapon during World War I to form irritating, dense smoke on the battlefield.
The exothermic reaction between aqueous NaBH4 and SnCl4 involves the borohydride reducing the Tin(IV) to Tin metal, forming BH3 and H2 gas in the process.
Source: https://youtube.com/chemicalforce
#ScienceGIF #Science #GIF #Chemical #Reaction #Rxn #Chemistry #Sodium #Borohydride #Aqueous #Tin #Tetrachloride #Powder #Solution #Air #Smoke #Reducing #Reduction View Original Post on Google+