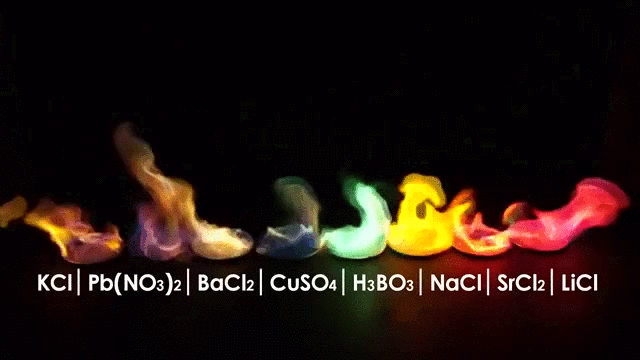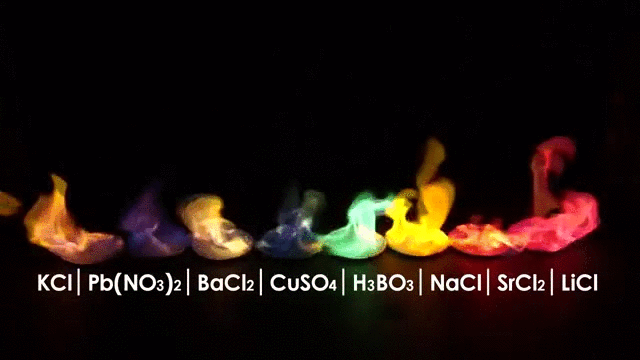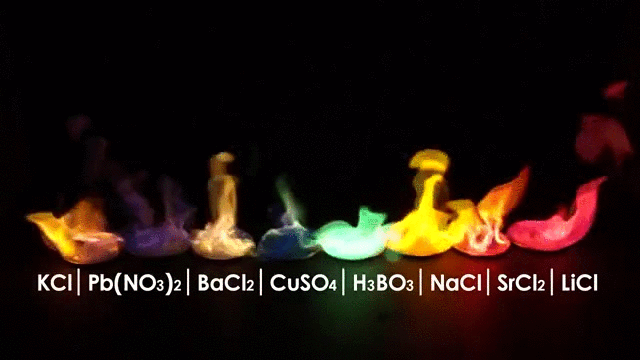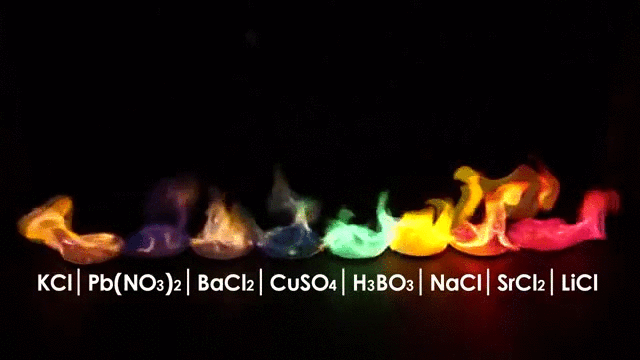The burning of different elements results in different flame colors. This occurs because the process of burning something excites the outer shell electrons of an atom to a higher energy shell. When the electrons fall back to their original position, they release energy in the form of heat and light. Because each element has a different number and arrangement of electrons, they emit different frequencies of light.
Source: https://youtu.be/NY-bnY0yjWw
#ScienceGIF #Science #GIF #Flame #Burning #Element #Molecule #Atom #Electrons #Heating #Color View Original Post on Google+