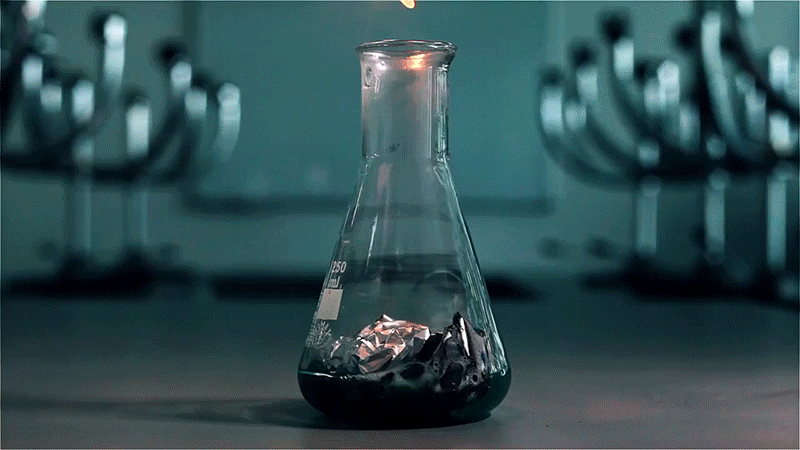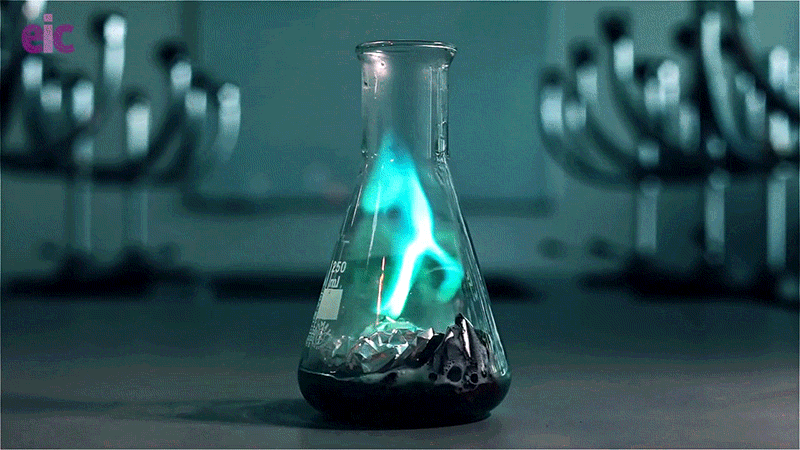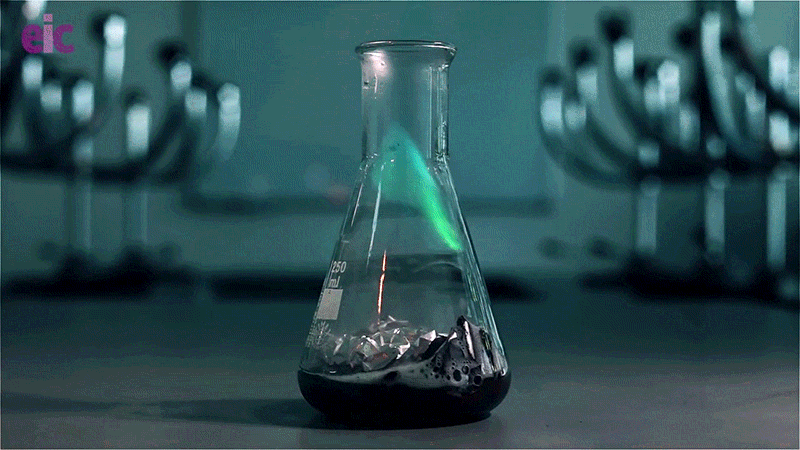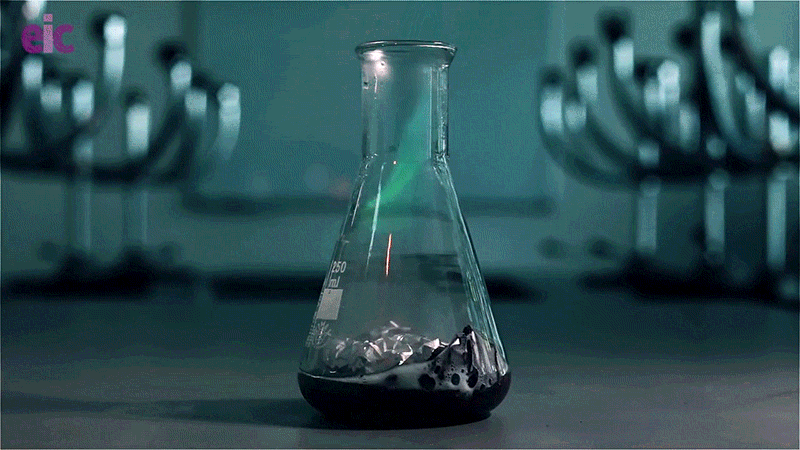Aluminium is the third most abundant element in the Earth’s crust and so highly reactive that native aluminium is actually quite rare. When exposed to air, a thin layer of aluminium oxide forms on the surface and prevents further reaction.
In this experiment, a solution of copper(II) chloride and hydrochloric acid is poured over a piece of aluminium foil. The chloride ions disrupt the aluminium oxide layer and react with the raw aluminium metal to produce hydrogen gas. Igniting the gas results in an eerie green flame caused by the copper released during the reaction.
Source: http://www.rsc.org/eic/2014/09/aluminium-oxide-chloride-reaction
#ScienceGIF #Science #GIF #Chemistry #Reaction #rxn #Aluminium #AluminiumOxide #Chloride #Flame #Gas #Hydrogen View Original Post on Google+