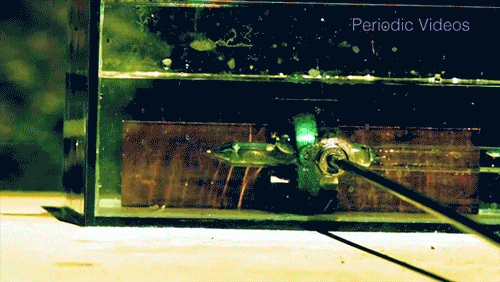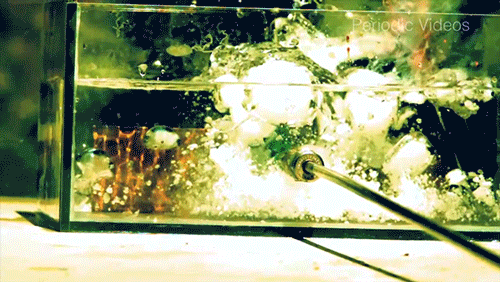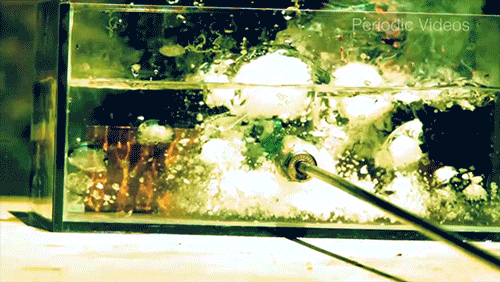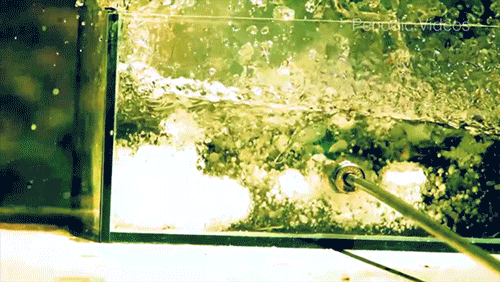
Caesium (Cs) is a soft, silvery-gold alkali metal that reacts violently when exposed to water. The reaction is extremely exothermic and produces caesium hydroxide (CsOH) and hydrogen gas (H2). The reaction occurs so quickly that it will often shatter its container, as seen by the enormous force warping the walls of this plexiglass box.
The caesium is initially contained in a glass vial that is shattered by the metal rod to expose it to the water.
Source: https://youtu.be/b2YrZNahqiw
#ScienceGIF #Science #GIF #Caesium #Water #Reaction #ChemicalReaction #Exothermic #TestTube #Hydrogen View Original Post on Google+