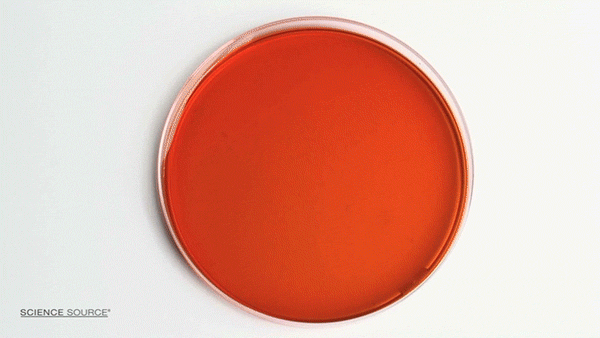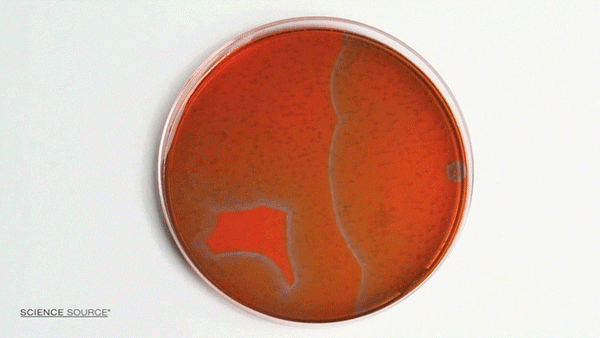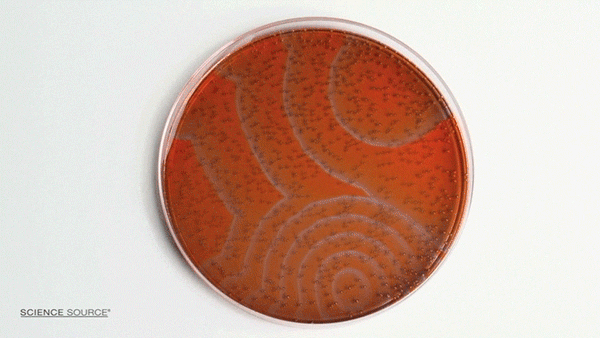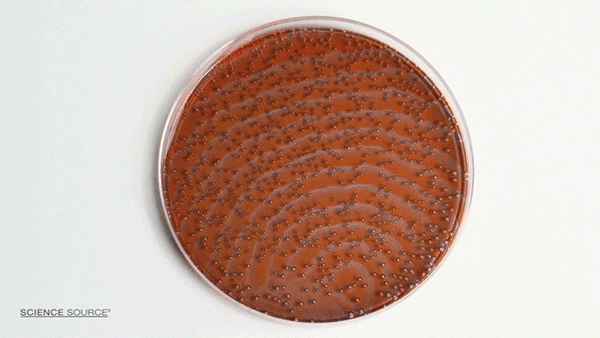
The Belousov-Zhabotinsky (BZ) reaction is a classic example of non-equilibrium thermodynamics that results in a nonlinear chemical oscillator. The colors are caused by the indicator chemical ferroin that appears red when its iron atom is in the Iron (II) state and blue when in the Iron (III) state. The varying concentrations of the reactants result in regions of localized scarcity which causes the appearance of traveling waves.
Source: https://vimeo.com/132128924
#ScienceGIF #Science #GIF #Reaction #Chemical #Oscillating #Chemistry #Thermodynamics #Equilibrium View Original Post on Google+